Spectra of stars
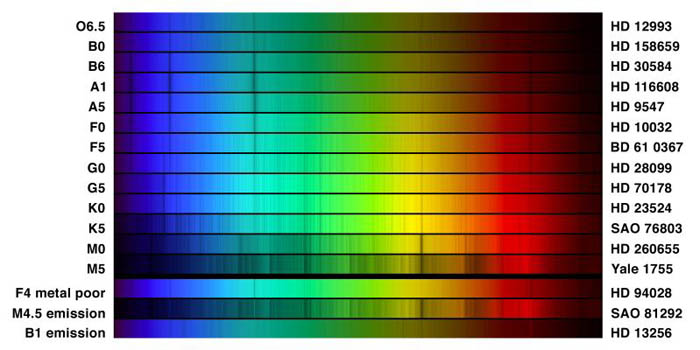
"This picture compares the spectra of different classifications of star, in the visual range from 400 to 700 nanometers (4000 to 7000 Angstroms). Thirteen regular types are shown, and at the bottom are three special cases, all selected from the spectrophotometric atlas by Jacoby, Hunter and Christian, 1984, which used data from the Kitt Peak National Observatory's 0.9-meter telescope."
- National Optical Astronomy Observatory
 Larger image [1,075 KB]
Larger image [1,075 KB]
- National Optical Astronomy Observatory
Notes in a nutshell

German postage stamp commemorating Fraunhofer's discovery of dark lines in the spectra of the Sun.
-
Why doesn't the Sun emit just the spectra of hydrogen and helium?
Hot dense objects emit a continuous spectrum of light, regardless of their particular elemental composition by blackbody radiation. The Sun and Earth behave this way.
If all stars emit a continuous spectrum, i.e. all colors, wouldn't their emitted spectra be identical and useless in distinguishing differnces in composition? Yes. But what we see is not the spectra directly from the surface of the Sun, but the spectra after it has passed through the Sun's "atmosphere".
When you look at the spectra of light directly from the disk of the Sun, you are looking at the spectrum produced by blackbody radiation minus any lines of color that have been snagged by absorption by intervening gases.
To see emission lines of hydrogen and helium, you need to look at light emitted from areas of gas that are not overwhelmed by light from blackbody radiation, i.e. not looking directly into the Sun.
-
If you look at the solar spectra in detail there are missing lines of light that have been absorbed by intervening matter, mainly hydrogen and helium.
-
When light is absorbed, the electrons in an atom are energized to jump from one obtial to a higher one. The electrons around an atom are sensitive to absorbing very particular quantities of energy from light. Each photon of light contains a unique quantity of energy. Colors towards blue contain more energy and colors towards red contain less energy. Many different kinds of electron jumps are possible between different levels, and each jump is triggered by a unique frequency (also unique color and energy) of light.
We know which colors of light are absorbed by each possible electron jump in each element based on lab experiments. By matching the missing lines in a star's spectrum with the known absorption lines for each element, voilá! We know a star's composition.
Note though - electrons eventually fall back to lower energy states and give up the energy as light once again, but the viewer still doesn't see these lines of color in spectra. Why?
When electrons drop back and re-emit energy, the energy isn't necessarily emitted in the same frequency (color), or the same direction it was going before. This makes colors in these frequencies much weaker and nearly absent to the viewer's perspective.
-
We've been talking about the color of visible light out of convenience, but this all applies to the invisible parts of the spectrum beyond the visible too.
-
You can use spectra to estimate the temperature of the star. Each star doesn't emit the same proportion of energy in each color. Most of the Sun's energy is concentrated towards yellow. Objects that are hotter emit more energy towards blue, and cooler objects emit more energy towards red - even though all the colors are emitted to some degree in continuous spectra.
-
The spectra of objects that are moving away from you are shifted towards red. Like sound, light is Doppler-shifted depending on whether the object is moving towards or away from the viewer or listener. Approaching sound rises in pitch or frequency when it approaches the listener and drops in pitch when it moves away. The same thing happens to light. Approaching objects shift in color towards blue and receding objects shift towards red.
-
Thus, it is important to understand all of the significant factors that influence the spectra of stars before using spectra to determine composition, motion, temperature, or other characteristics of stars.
Related terms: Blackbody radiation, Planck curve, Wien's law, Stefan-Boltzmann law, Wien's displacement law, Fraunhofer lines, Kirchoff lines,
See related discusion at Physics Forums
See related discusion at Ask-a-Scientist
Questions for thought
- What does the prism do that is so useful?
- If the Sun is made up mostly of hydrogen and helium, why doesn't the spectra look like the emission spectra of hydrogen and helium?
- Does the Sun emit the same quantity of radiation at each color (frequency)?
- How can we tell the temperature of stars?
- How can we tell whether stars are moving towards or away from us using their spectra? What is the Red Shift?
- Are stars emitting and absorbing radiation outside of the visible light spectrum?
- If you were building a solar collector, would you want to find a material that greater or fewer colors of light?
An unexpected discovery could yield a full spectrum solar cell
Lawrence Berkeley National Laboratory - What does the spectrum of an regular incandescent light bulb look like? Fluorescent light?
Related resources
-
Spectroscopy: Unlocking the Secret in Starlight
Explains how spectra are produced by stars and how this information is used to study stars. Excellent resource for AP physics students and science teachers.
Australia Telescope Outreach and Education
-
Spectral Classification of Stars
University of Nebraska
-
High Resolution Solar Spectrum
Detailed view of our Sun's spectrum in the visual range.
National Optical Astronomy Observatory
-
High resolution spectrum of Arcturus
Detailed view of the star Arcturus in the visual range.
National Optical Astronomy Observatory
-
Solar and Heliospheric Observatory
"a project of international collaboration between ESA and NASA to study the Sun from its deep core to the outer corona and the solar wind." Breathtaking images and movies of our Sun.
Solar and Heliospheric Observatory
-
Emission and absorption lines of different elements
Department of Physics, University of Oregon
-
What do spectra tell us?
Goddard Space Flight Center, NASA
