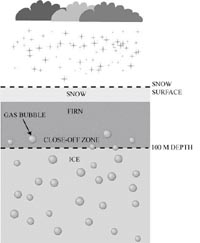
Schematic diagram illustrating the mechanism by which air trapped between snow flakes falling in polar regions is slowly trapped in bubbles as the snow is compacted into ice. The ice, which extends for hundreds of feet down, can be drilled using special techniques and taken back to a laboratory. The air, as well as the ice itself, can be extracted from the ice core and chemically analyzed. (After: Delmas R.J., 1992)
