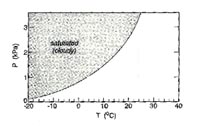
The saturation of air with water vapor depends on both its temperature (on the x-axis) and water vapor pressure (on the y- axis). The water vapor pressure is a measure of the amount of vapor in the air. At higher temperatures, much more water has to be present in air before clouds can form.