
Typical marine phytoplankton – (A) dinoflagellates, (B) diatoms, (C) coccolithophorids
 Typical marine phytoplankton – (A) dinoflagellates, (B) diatoms, (C) coccolithophorids |
(A) Thick Eocene radiolarians compared with (B) delicate recent ones. |
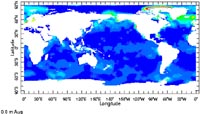 Productivity map of the surface ocean waters from satellite surveys for August. The dark blue color that represents most of the ocean area indicates low productivity (i.e. low plankton). The lighter blue color in the equatorial upwelling and coastal upwelling areas indicate higher productivity (i.e higher plankton). Greens, yellows to red color zones indicate even higher productivity (the colors in the high artic are an artifact of the measurement technique, and do not indicate high productivity in the arctic region.) |