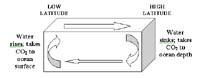
Sketch illustrating the concept of vertical deep mixing, where carbon dioxide is transported from the ocean surface to the ocean depths by sinking cold water in the high latitudes. If brought to the surface (for instance, by upwelling) the cold water will warm up and release some of its carbon dioxide to the atmosphere.