
“Good” Ozone is found in the stratosphere. “Bad” Ozone is a pollutant in the troposphere. For more information on “Good” and “Bad” Ozone, see the Environmental Protection Agency’s Web site:Environmental Protection Agency
 “Good” Ozone is found in the stratosphere. “Bad” Ozone is a pollutant in the troposphere. For more information on “Good” and “Bad” Ozone, see the Environmental Protection Agency’s Web site:Environmental Protection Agency |
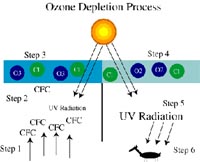 Schematic of the ozone depletion process in the stratosphere, Steps 1-6 are explained in detail in the text below. |